- Home
- /
- Analytics
- /
- Stat Procs
- /
- Re: Non-normal data; PROC GLIMMIX
- RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
Completed a feedlot Trial with 6 treatments (animal was exp. unit). We collected blood (twice) and analyzed multiple blood serum components (e.g., urea N, glucose, etc.).
Some of the continuous variables are not normally distributed.
Right now, I have the following SAS statement together, but need help on determining exactly what to use (run all of them and look at model with best fit?):
"Method = " laplace, quad or ?
"Dist = " normal, gamma, invgauss, beta, or ?
"Link = " log or ?
"Adjust = " How do you know to use "Tukey" or "KR"?
I believe that ARH(1) will provide the best fit, but... do I need to also try CSH, AR(1), CS, ...?
There are too many possible combinations of the above factors, thus I'm sure I'd screw up somewhere.
Guidance on what to use would be much appreciated.
I attached an Excel spreadsheet of the data if needed (I highlighted the serum variables that I can't get "normal"; through data transformation, deleting any outliers that I can, etc...).
PROC GLIMMIX method=laplace;
CLASS TRT DAY ID;
MODEL GLUCOSE = TRT day trt*day/dist=negbin link=log;
Random day / subject = id(trt) type =ARH(1);
LSMEANS TRT day/PDIFF ADJUST=TUKEY;
LSmeans trt*day/slicediff=day adjust=tukey;
RUN;QUIT;
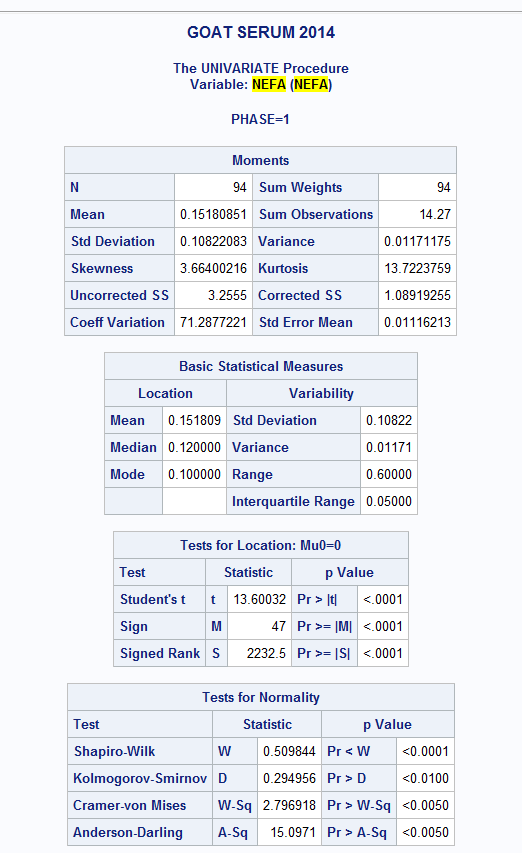
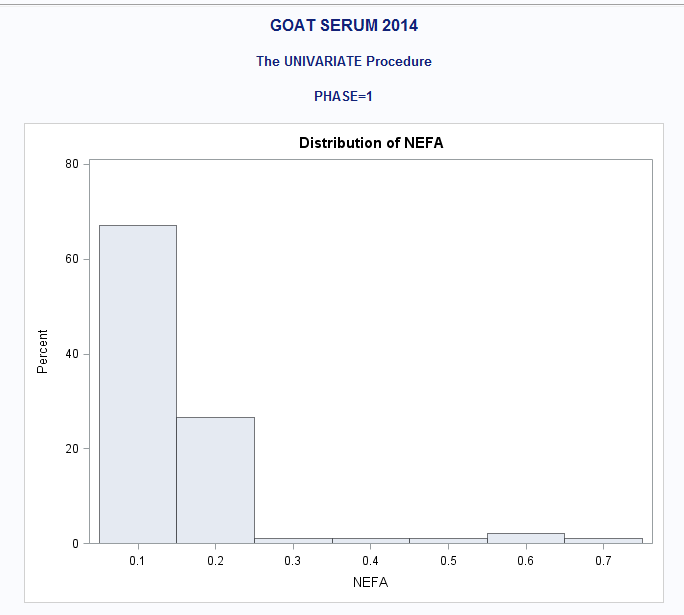
ALSO: PROC UNIV. of NEFA if it helps any

Accepted Solutions
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
I would say some of the endpoints have a normal distribution of errors (remember that is the assumption, NOT that the endpoint itself is normally distributed) The others are probably best fit with a lognormal distribution.
As far as the error structure, a lot depends on the spacing of the measurements in time. AR(1) and ARH(1) assume that the measurements are equally spaced in time. CS and CSH assume that the correlation between close together time points is the same as more separated in time points. However, you only have two time points (Day 0 and Day 26), so CSH and CS would make perfect sense. Fit both, pick the one that yields the smaller corrected AIC value. Try the following:
PROC GLIMMIX data=yourdata;
CLASS TRT DAY ID;
MODEL GLUCOSE = TRT day trt*day/dist=lognormal ddfm=kr2;
Random day /residual subject = id(trt) type =CSH;
LSMEANS TRT day/DIFF ADJUST=TUKEY;
LSmeans trt*day/slicediff=day adjust=tukey adjdfe=row; /* I really dislike Tukey's adjustment for repeated measures data. You might look into adjust=simulate */
ODS OUTPUT lsmeans=lsmeans;
RUN;QUIT;
Now, to get lognormal estimates back onto the original scale you'll have to post-process the lsmeans dataset, and this is where some mathematical statistics enters the picture. Simply exponentiating the estimate will give an estimate of the median value. If you want to get an estimate of the expected value and of the standard error of the expected value, you'll need the following code:
data btlsmeans;
set lsmeans;
omega=exp(stderr*stderr);
btlsmean=exp(estimate)*sqrt(omega);
btvar=exp(2*estimate)*omega*(omega-1);
btsem=sqrt(btvar);
run;
Steve Denham
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
I would say some of the endpoints have a normal distribution of errors (remember that is the assumption, NOT that the endpoint itself is normally distributed) The others are probably best fit with a lognormal distribution.
As far as the error structure, a lot depends on the spacing of the measurements in time. AR(1) and ARH(1) assume that the measurements are equally spaced in time. CS and CSH assume that the correlation between close together time points is the same as more separated in time points. However, you only have two time points (Day 0 and Day 26), so CSH and CS would make perfect sense. Fit both, pick the one that yields the smaller corrected AIC value. Try the following:
PROC GLIMMIX data=yourdata;
CLASS TRT DAY ID;
MODEL GLUCOSE = TRT day trt*day/dist=lognormal ddfm=kr2;
Random day /residual subject = id(trt) type =CSH;
LSMEANS TRT day/DIFF ADJUST=TUKEY;
LSmeans trt*day/slicediff=day adjust=tukey adjdfe=row; /* I really dislike Tukey's adjustment for repeated measures data. You might look into adjust=simulate */
ODS OUTPUT lsmeans=lsmeans;
RUN;QUIT;
Now, to get lognormal estimates back onto the original scale you'll have to post-process the lsmeans dataset, and this is where some mathematical statistics enters the picture. Simply exponentiating the estimate will give an estimate of the median value. If you want to get an estimate of the expected value and of the standard error of the expected value, you'll need the following code:
data btlsmeans;
set lsmeans;
omega=exp(stderr*stderr);
btlsmean=exp(estimate)*sqrt(omega);
btvar=exp(2*estimate)*omega*(omega-1);
btsem=sqrt(btvar);
run;
Steve Denham
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
Exactly what I needed. You saved me a couple of day and a few headaches.
MANY THANKS!
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
In your post below, I am curious:
What is the difference in your editor statement vs. iLink function in an LSMeans statement?
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
Because the variance is separable from the mean for the lognormal distribution, using the ILINK option with DIST=LOGNORMAL will return the same value on both the link transformed and original scales. That is why you have to post-process.
Steve Denham
- Mark as New
- Bookmark
- Subscribe
- Mute
- RSS Feed
- Permalink
- Report Inappropriate Content
how do I get beta (PROC GLIMMIX Beta distribution) back on original scale?
April 27 – 30 | Gaylord Texan | Grapevine, Texas
Registration is open
Walk in ready to learn. Walk out ready to deliver. This is the data and AI conference you can't afford to miss.
Register now and save with the early bird rate—just $795!
ANOVA, or Analysis Of Variance, is used to compare the averages or means of two or more populations to better understand how they differ. Watch this tutorial for more.
Find more tutorials on the SAS Users YouTube channel.


